Particle and droplet size of aerosols determine the effectiveness of inhalation therapy
Inhalation therapy involves an active substance, or a combination of active substances, being administered as an aerosol through the airways. The nebulizer or inhaler device is specifically designed to provide the patient with defined drug amount for inhalation. The area of the respiratory tract that the drug substance is delivered to is dependent on the particle or droplet size; by effectively controlling particle size, a specific area can be targeted, where the medicine will take effect or be absorbed.
In general, inhalation therapy is divided into two main types of usage. In nebulizer applications, a larger amount (1-5 ml) of a pharmaceutical suspension or solution is dispersed mechanically, e.g. atomization using pressurized air or ultrasound. This aerosol is then inhaled for several minutes. The other, more common, method delivers a metered amount of drug in a short burst, which can be inhaled in a single breath. This aerosol burst can be generated by propellant, mechanical energy or the turbulent flow of inhaled air.
These two applications pose different challenges towards the measuring system; the first case requires a continuous measurement of the proportion of respirable droplets at high concentrations for several minutes. In the second case the respirable fraction, as well as the quality of the dispersion, need to be characterised in just a fraction of a second, and for doses in the micro or milligram range.
Optimising inhalation therapy to be able to use the minimum required active ingredient quantity requires a compromise; on the one hand the particle generated must be designed to take into account a wide variety of parameters in order to fulfil its intended purpose, but on the other hand the drug must be administered by an inhaler device that is repeatable and easy-to-use.
The use of the "Anderson Cascade Impactor" (ACI) or the "Next Generation Pharmaceutical Impactor" (NGI) is common practice for both product development and quality assurance testing for inhaler devices and formulations. The use of these devices is frequently stipulated by the US and EU pharmacopoeia. These analytical methods typically provide 8 to 10 fractions of the given formulation based on fluid mechanical separation. The division of the fractions is not always clear, as the boundaries between the classes in the analysis are dependent on the flow rate to which the impactor is calibrated. Due to the large surfaces of the plates, it often requires multiple doses to achieve valid measurements. In addition, the wet-chemical evaluation of the results is very time-consuming, labour intensive and is frequently subject to anomalous.
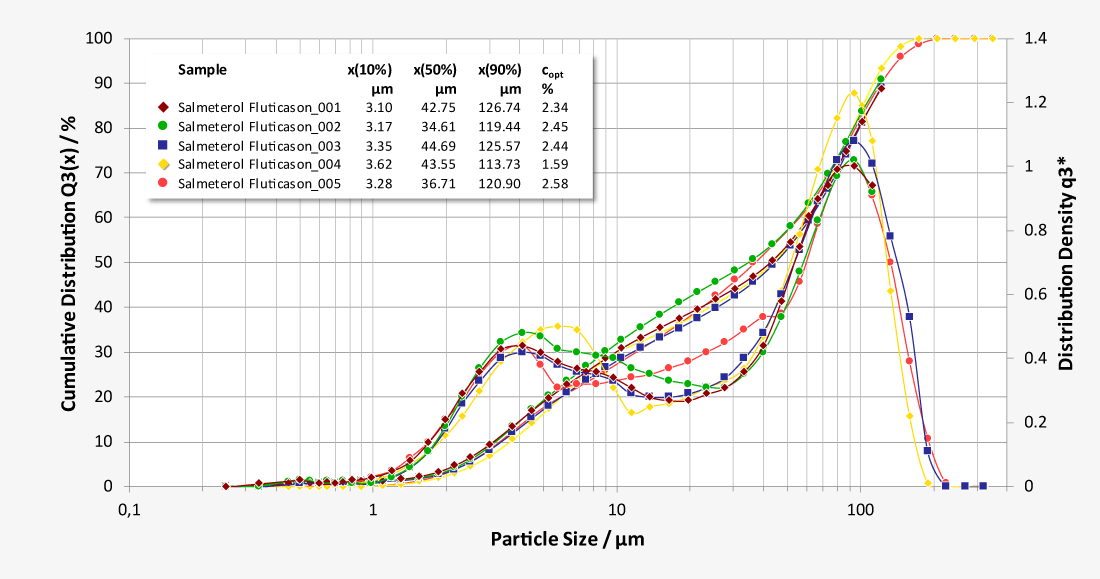
Characterisation of pharmaceutical inhalants with laser diffraction
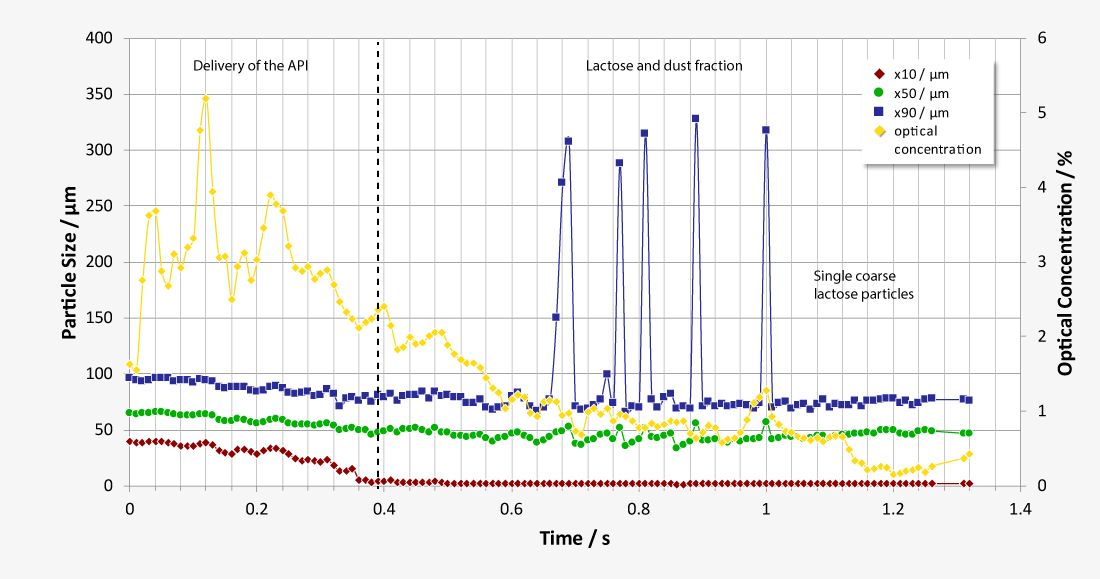
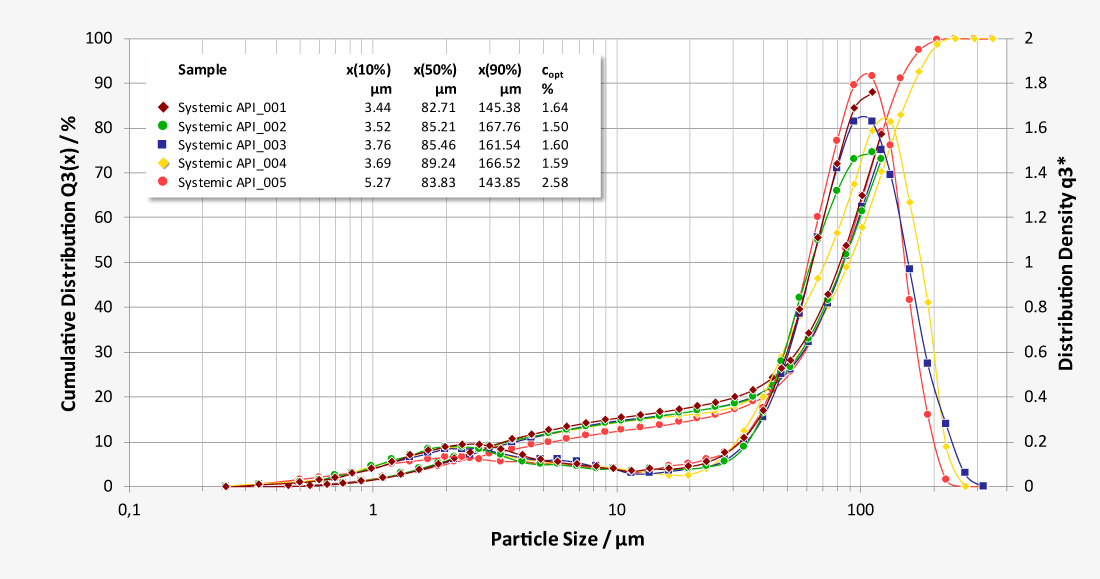
Laser diffraction offers an alternative approach to limit the number of NGI or ACI analysis required. As a purely optical method, laser diffraction offers measurement times of just a few seconds with a resolution of 18 classes below 10 µm, while at the same time classifying the carrier substances. Thus, laser diffraction measurements enable both quantification of the respirable fraction of the active pharmaceutical ingredient (API) and correlation of the results with NGI and ACI measurements at comparable flow parameters. In addition, the high data acquisition rate of up to 2,000 complete readings per second allows investigations of the release of the inhalant under dynamic aspects at almost any flow rate, as a flow-dependent calibration is not required. Essential parameters such as flow rate and pressure drop are controlled by the software and the ambient temperature and relative humidity are recorded.
Due to its modular design the INHALER system can be adapted to almost any pressurised metered dose inhaler (pMDIs), single or multidose dry powder inhaler (DPI), nebulizer or Fine mist inhaler (Soft Mist ™ Inhalers | SMI). This flexibility means that by combining the HELOS/BR laser diffraction sensor with the INHALER unit, opens up considerable savings as well as development potential for the user. In addition, using the INHALER device in parallel with our established dry powder dispersion technology in the expanding DPI area can also make a considerable contribution to characterising the performance and optimisation of the device or formulation combination.
During inhalation, the active ingredient needs to disconnect itself from the carrier in order to reach the lungs. Therefore, the inter-particular interaction needs to be strong enough to fixate the active ingredient to the carrier during the production, but weak enough that it disconnects during the inhalation procedure as completely as possible. Appropriate DPI designs must be capable of producing the required particle size in order to reproducibly deliver the intended dosage and size distribution. Particles within the size range of 3 to 5 µm get deposited primarily in the central parts of the lungs, where they take effect within the bronchi. In the size range of 1 to 2 µm they penetrate the alveoli as anti-inflammatory active ingredients. Particles of an even smaller size are ineffective, because they get resorbed systemically or exhaled.
During the development of medical aerosol generators (MAG), standard procedures have been established. One of them is the determination of the aerosol’s aerodynamic particle size distribution. The chosen flow rate has a significant influence on the size distribution and gets coordinated with the patients normal breathing. The dose absorbed by the patient is another important value to be determined.
When combined with the modular INHALER adapter, the compact laser diffraction sensor HELOS/BR provides time-resolved and reproducible analyses of size distributions of droplet mist and solid aerosols with particles ranging from 0.25 µm up to 875 µm. The INHALER adapter’s modular setup offers optimal possibilities for adaptation to fit different types of pMDIs, DPIs, nebulizers or recent inventions like Soft Mist™ Inhaler. To simulate different breathing profiles, measurements can be implemented depending on volume flow rate or absolute pressure drop. The inhalation and measurement duration are freely selectable.
- Adaptability of measuring system to different applicators of DPIs, MDIs, SMIs and nebulizers
- Setting and monitoring of air flow parameters within the dosing system according to inhaling processes and medical requirements
- Flexible geometry of dosing unit to simulate different inhalation scenarios/parts of airways
- Possibility to eliminate coarser particles to simulate function of upper airways
- Separation of measured particles, by downstream impactors for example, for subsequent gravimetric investigations
Download application notes for detailed information
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Inhalers and nebulizers, routinely tested using and partially developed on the INHALER unit include: Aerolizer®, Cyclohaler®, Turbuhaler®, Twincer®, Twisthaler®, Novolizer®, Respimat®, HandiHaler®, Diskus®, Jethaler®, Easyhaler®, RotaHaler©, Aeroneb®, TurboBOY®, NebuTech®HDN®, belAir®, AeroEclipse®, MicroDrop® et al.
Application strengths
- Open measuring zone in parallel laser beam enables measurement within the free aerosol stream
- Modular set-up of dosing system for a wider application range
- High adaptability to different types of inhalers
- Possibility for additional use of impactors for complementary analyses like mass determination
- Characterisation of delivery and the dosage homogeneity
- High time resolution of a single application with up to 2,000 measurements per second
- Recording of volume flow, ambient temperature and relative humidity
- HELOS & RODOS can be used for analysis of formulation and single components, independent from the inhaler | Direct comparison to INHALER analysis
Customer benefits
- Reliable determination of “Fine Particle Fraction” from pharmaceutical preparations for the inhalation
- Dispersion control during new formulations
- Chemical analysis of particle fractions in combination with cascade impactors
- Quality control during method takeover
- Validation of results within pharmaceutical context
- High reproducibility and repeatability of measuring results
- Considerable saving and development potential through combination of formulations with different devices: MDI, DPI, nebulizer or SMI
- Fast measurement with minimal preparation and minor need for cleaning | 3 min per analysis
- Modular measuring system expandable for changing applications in the pharmaceutical development (powder, granules, suspensions, emulsions …)