Detection of fine and coarse droplets in pharmaceutical products
In pharmaceutics, particulate systems are used as active ingredient carriers. The precise characterisation of these systems is a prerequisite to the product being approved. The particle size distribution has a significant influence on the effect of the pharmaceutical product and is used as an indicator of contaminations. Pharmaceutical emulsions such as eye drops are subject to various quality requirements. In eye emulsions or suspensions, the bioavailability (i.e. the residence time and duration of the effect, the rate of release and the absorption of the eye drops) depends on the particle size of the disperse active ingredient. In addition, overly coarse active ingredient particles can irritate or damage the eye.
The use of PCCS technology means it is possible to measure eye emulsions and suspensions that are very often turbid without extensive sample preparation. NANOPHOX in its original form analyses the difluprednate ophthalmic emulsion shown here as an example for relieving pain and swelling after eye surgery. Dilutions that may alter chemical properties and contaminate the sample can be dispensed with. The measuring system’s stable and easy-to-use measuring method is ideal for research, production and quality control for pharmaceutical emulsions.
Particle size distribution of difluprednate ophthalmic emulsion
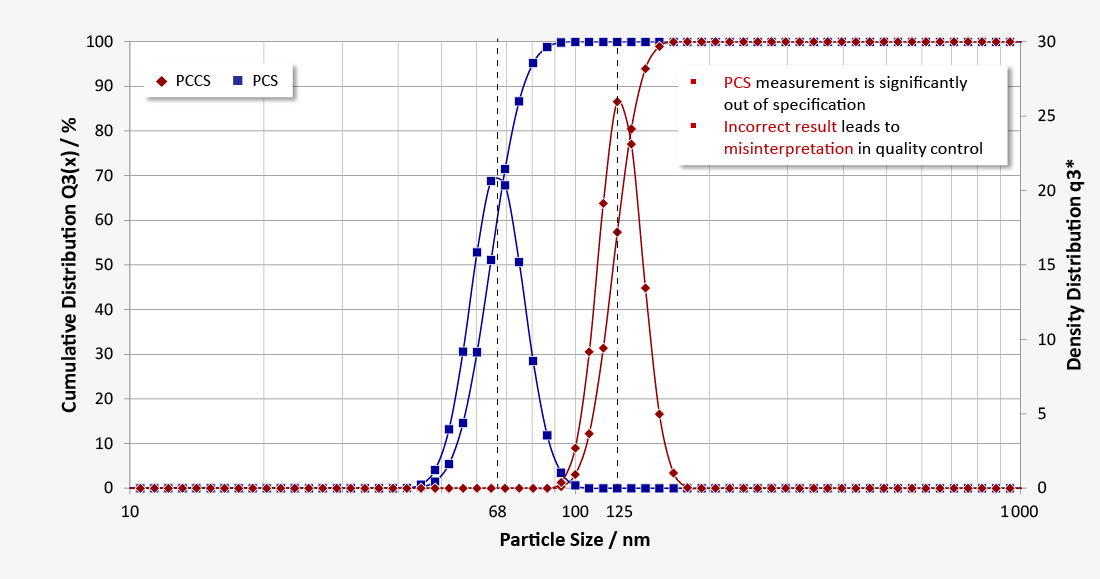
In the measurement of this sample with PCCS, the result shows the expected monomodal size distribution with a mean hydrodynamic diameter of 125 nm. The measurement in PCS mode, however, results in an erroneous value of 68 nm; this can be explained by the influence of multiple scattering. Such measurement errors lead to misinterpretations in the quality control and can be successfully avoided by using the PCCS evaluation.
- Evaluation of the formulation with respect to stability, bioavailability and safety
- Measurement of the particle size and distribution for pharmaceutical drug safety and efficacy
- Analysis of the ophthalmic emulsion at high concentration
- Analysis of the long-term stability
- Support for the research and development of new, innovative pharmaceutical products by optimising the physical properties of particle systems
Download application note for detailed information
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Application strengths
- Measurement of particle size distribution regardless of the particle concentration
- Low to no sample preparation
- High reproducibility and repeatability of the measurement results
- Simple and stable measurement method Analysis of the droplet size in original concentration
- Reliable measurement of the stability through cross-correlation amplitude and mean diameter depending on the time
Customer benefits
- Reduction of measurement errors due to less complex sample preparation
- Ensuring compliance with official regulations
- Compliance with the requirements of 21 CFR Part 11 for pharmaceutical products
- Development of disperse particle systems with desirable, controlled properties
- Reliability over period of use of ophthalmic emulsion
- Reduction of the recipe development time












