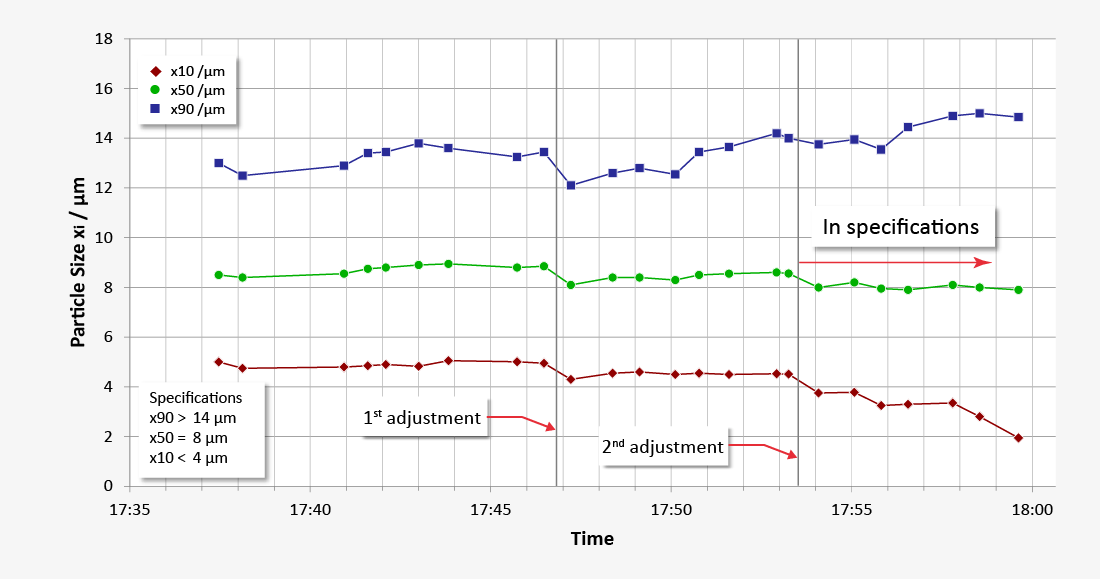
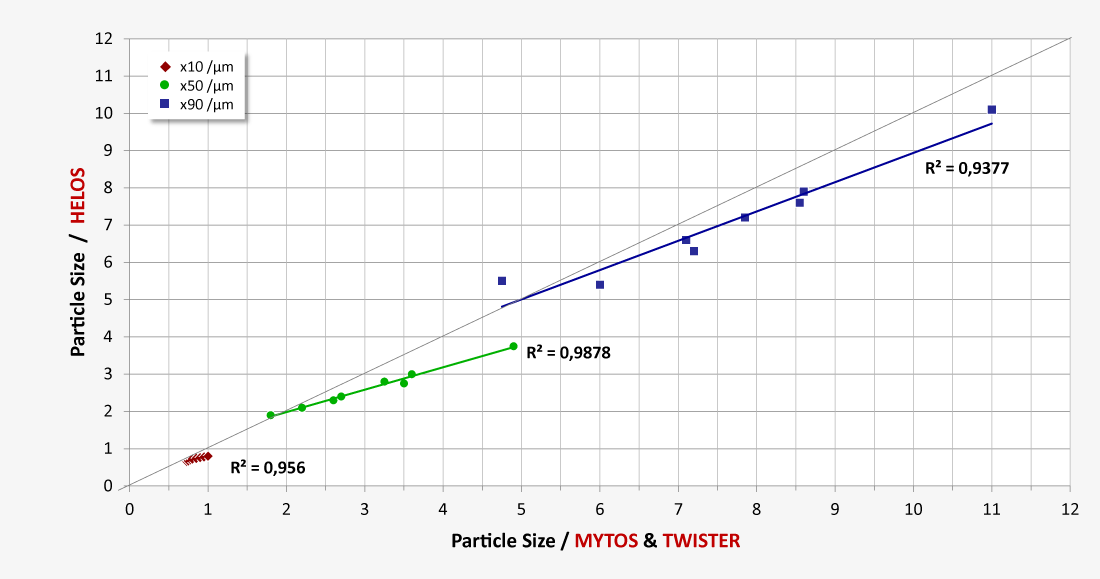
Optimizing the quality of lactose in laboratory or process environments with laser diffraction particle size analysis
Lactose is a white, crystalline slightly sweet tasting powder. It is a disaccharide consisting of glucose and galactose and is widely used as an excipient in the pharmaceutical industry (filler, binder). Lactose powders are manufactured by selectively modifying the size, shape and size distribution of particles and they have special characteristics in terms of compactability, flow behaviour and granulability.
Lactose is a natural carbohydrate constituent of milk, and is therefore also referred to as milk sugar. The whey required to make lactose is obtained as a by-product of cheese manufacturing. By concentrating the whey, lactose is made to crystallise, and is then filtered, washed and dried. Depending on the intended use, different raw qualities with varying purity and content are manufactured. Pharmaceutical grade lactose contains more than 99.5% lactose monohydrate with appropriate purity.
In the formulation of oral solid dosage forms or dry powders for inhalation, pharmaceutical grade lactose is used in many different particle size distributions and modifications. The compressibility, flow characteristics and granulability of the lactose powder depend directly on the size, shape and size distribution of the particles. These physical attributes are selectively adjusted by sieving, milling, spray-drying or agglomeration to guarantee the best functional characteristics for tableting, granulation or blending processes. Fast and reliable particle size analysis ensures that the quality of the various lactose products is consistently high.
Better particles with best instruments
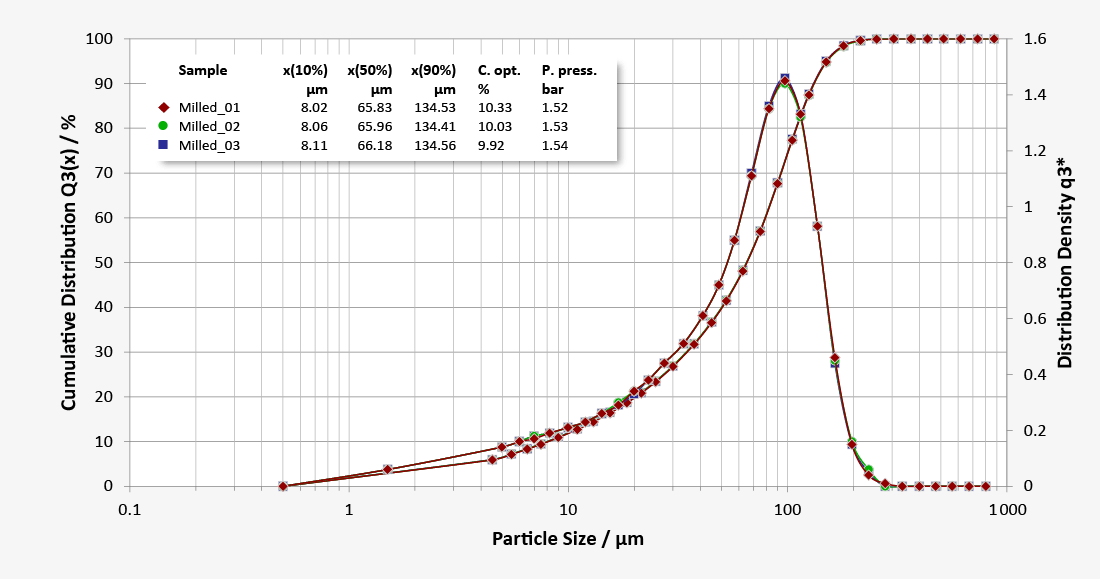
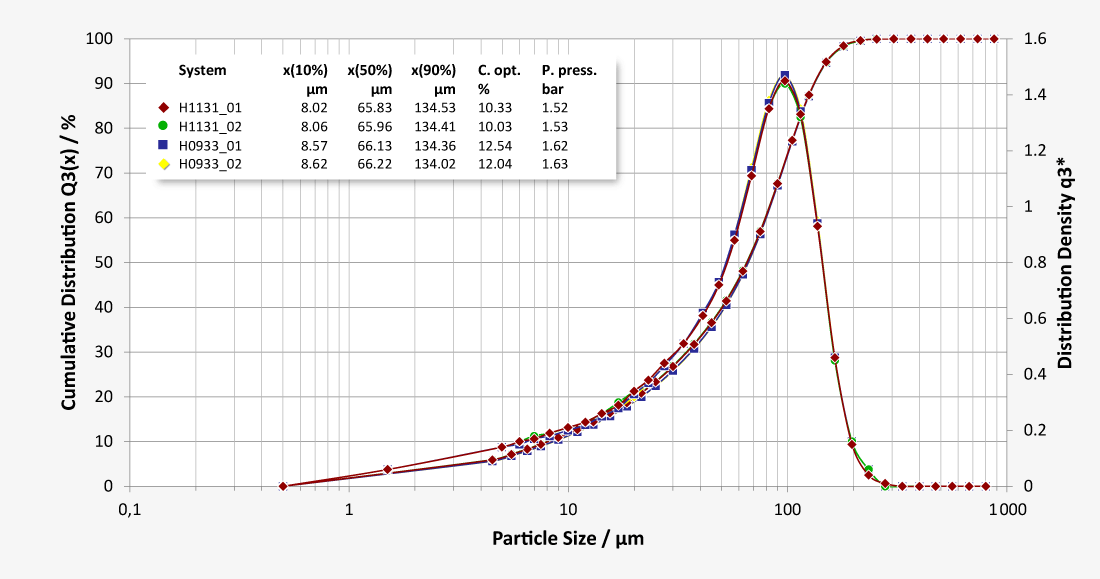
Our laser diffraction systems ensure reliable, precise and fast monitoring of the particle size distribution of all lactose powder products, both for quality control purposes in the lab and also in the process itself during high-volume production. The RODOS dry dispersion technology also guarantees reproducible dispersion of larger sample quantities in order to obtain statistically reliable measurement results. Our robust dry method delivers outstanding repeatability of individual measurements and unparalleled comparability of measurement results from system to system, also at production facilities in different locations. Measurement methods developed for different product qualities can easily be transferred to other systems as Standard Operating Procedures (SOP). Thus consistent high qualities and batch-to-batch consistency are ensured by precise monitoring, which justifies the confidence in the premium quality of the products.
Pharma-oriented instrument design, with fast and easy to clean components, outstanding availability and long service life, ensures optimum use and long-term value of the systems. Our highly qualified service employees provide support in preventative system care and maintenance, re-certification and qualification. Manufacturers of pharmaceutical excipients and innovative research laboratories as well as global pharmaceutical manufacturers and specialists with demanding formulations trust our expertise.
- Ensuring consistent high quality from batch to batch
- Fast and straightforward dry measuring method
- Excellent repeatability
- Excellent system-to-system comparability
- Minimal sample preparation work
- Fast and easy cleaning of parts in contact with the product
- Production control with timely measurements (up to GMP-compliant in-process controls)
Lactose products at a glance
Sieved lactose …
… has a relatively narrow particle size distribution range due to the sieving process. Sieved products have good flow and blending properties and are used for sachet filling, capsule formulation or direct tableting.
Milled lactose …
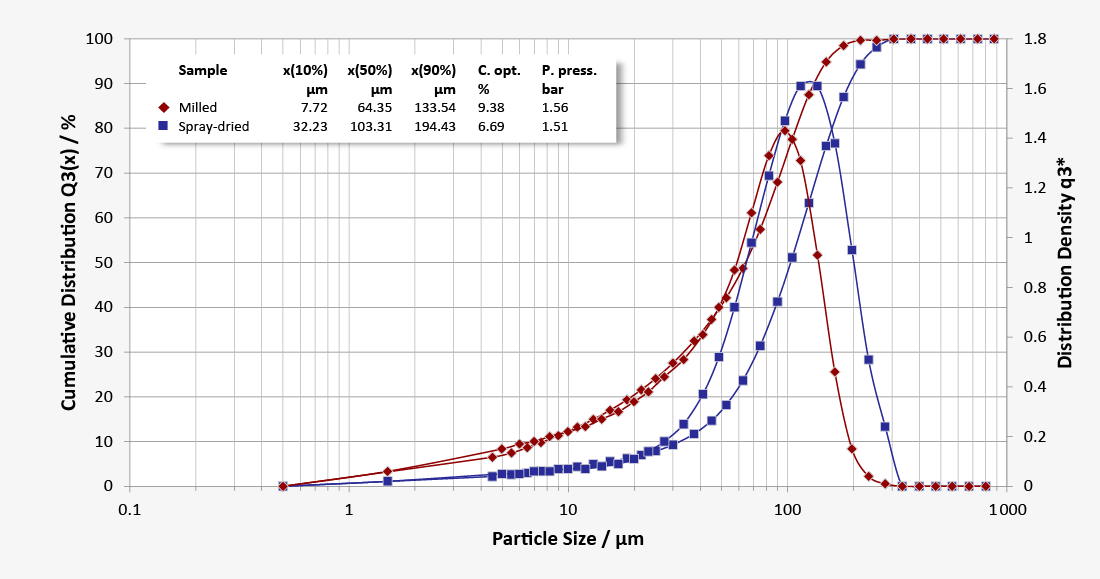
… is produced by milling to different degrees of fineness and particle size distributions. The fine, sharp-edged particles tend to be cohesive and show poor flow properties. Milled lactose is primarily used for granulation and subsequent tableting. The excellent compactability of fine milled lactose favours high tablet hardness.
Spray-dried lactose …
… has a relatively narrow size distribution of spherical agglomerates made up of fine crystals in a matrix of amorphous lactose. Spray-dried lactose is highly compactable, has outstanding flow properties and little, if any, dust content. This lactose quality is therefore suitable for filling sachets and capsules as well as formulations for direct tableting, or for improving the flow properties of poor flowing active ingredients.
Granulated lactose …
… consists of lactose monohydrate agglomerates that are built from fine lactose particles in a wet granulation process. Granulated lactose has the flowability of coarse crystals and the good compressibility of fine milled lactose. It is ideal for direct tableting due to its excellent miscibility and negligible dust content. The narrower the particle size distribution range, the greater the flowability of the lactose product.
Micronised lactose …
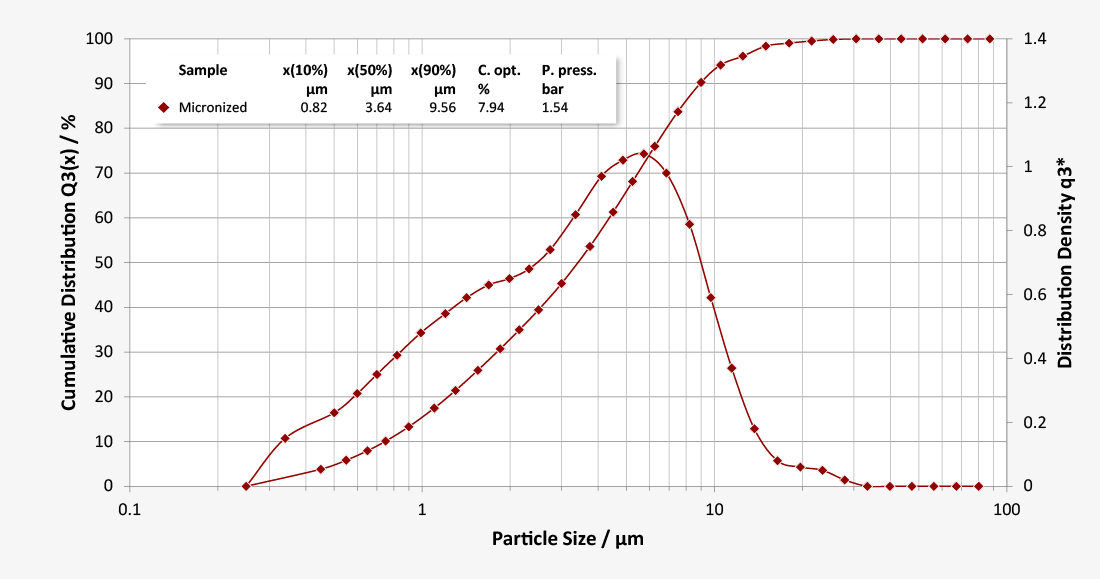
… consists of very fine milled lactose crystals, more than 90% of which are finer than 10 µm. Due to its cohesive properties, micronised lactose is ideal for pre-blending with an Active Pharmaceutical Ingredient (API) of similar particle size in order to effectively prevent a formulation from segregating. Extremely fine lactose powders also play a central role in the formulation of Dry Powder Inhalers (DPI) due to their effect on fluidisation and nebulisation.
Lactose for inhalation
Lactose is the most important carrier substance for formulations for pulmonary administration as Dry Powder Inhalers (DPI). Depending on the active ingredient and type of inhaler, specific particle size distributions and blends are necessary to achieve the required functionality. In the formulation of inhalants, milled, micronised and sieved lactose qualities are used.
Download application note for detailed information
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Application strengths
- Unparalleled repeatability of dry measurement method
- Optimum dispersion and measurement of the finest particles
- Straightforward method transfer and outstanding system-to-system comparability
- Hardly any sample preparation plus easy cleaning
- High resolution and parameter-free evaluation
- Measurement of statistically significant sample quantities
- ASPIROS microdosing system for small sample quantities
Customer benefits
- Robust, standard measuring method for all lactose qualities
- Assurance of high batch-to-batch consistency
- Reliable method transfer
- Comparability of different locations
- Well-founded trust in the product quality
- Long system service life with low maintenance requirements
Application strengths
- Fast and reliable in-process quality control
- Representative sampling and proven dry measurement
- Excellent comparability of laboratory and process measurement results
- GMP-compliant instrument design
- ATEX-design available
- Low maintenance and cleaning requirements
Customer benefits
- Final product quality monitored via closed control loop system
- Assurance of high batch-to-batch consistency
- Greater insight into and control of manufacturing processes (Quality by Design)
- Real time measurement of critical quality attributes in-process
- Reduction of noncompliant batches and rejects
- Long system service life with low maintenance requirements