Particle technology is of central importance for development and manufacture of finished dosage forms. Quality-defining product parameters, such as bioavailability, dosing accuracy, dispersibility and also positioning and release of active ingredients, are determined to a large extent by the physical properties of the underlying disperse systems. Flow properties, dust behaviour, compressibility or bulk density of intermediate products and formulations made up of active ingredients and excipients depend on dispersity variables such as particle size or particle shape. For applications ranging from active ingredient synthesis, preformulation and manufacture of small pharmaceutical product quantities in early clinical phases through to manufacture of approved products on an industrial scale, particle characterisation technology plays an important part both in product development as well as in quality and process control.
Over three quarters of pharmaceuticals are marketed in solid form, e.g. as powders, granulates, tablets, pills or capsules. The starting materials and intermediate products are usually available in powder form, and the properties of these powders determine the further processing capability and quality of the final preparations. However, liquid and semisolid dosage forms such as suspensions, emulsions, ointments, pastes, creams or gels are also disperse systems and contain solid particles or liquid, dispersed phases as active ingredients or excipients.
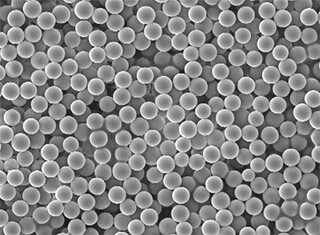
Particle size analysis is always required for solid dosage forms or for semisolid or liquid dosage forms with an undissolved active ingredient, i.e. forms containing disperse active ingredient particles. Typically, the particle size has a decisive influence on the physicochemical properties of the preparation in terms of bioavailability, solubility, dissolution rate, disintegration, uniform distribution of the active ingredient content and product stability. Analysis of the particle size or shape is recommended even if the following manufacturing steps are influenced by the characteristics of the particle size distribution or particle shape.
Droplet or particle size analyses are also performed for sprays, atomisers, inhalers (Dry Powder Inhalers (DPIs) and Metered Dose Inhalers (MDIs)) or nebulisers, which produce droplet or solid aerosols. Local deposition of particles in the respiratory tract and thus delivery at the intended site of action are determined by the particle size.
Sympatec technology for pharmaceutical development and manufacture
We offer innovative, high-performance measuring technologies to cover the entire range of applications for particle size and shape analysis in both the laboratory and manufacturing process: from small quantities as part of active ingredient development and preformulation through to manufacture of active pharmaceutical ingredients in batch operation or continuous volume processing on an industrial scale.
For over 30 years now, development and manufacture of our measuring systems have been based on the requirements and quality standards of the pharmaceutical industry. Our instruments reliably supply highly precise and reproducible results in very short measuring times and with excellent system-to-system comparability. The consistency of the evaluation methods guarantees conformity with the pharmaceutical product approval during the entire manufacturing phase even across equipment generations. A team of highly-qualified service engineers provides support for Sympatec instruments worldwide, and can also perform preventive maintenance and assist in system qualification. Instrument design tailored to the needs of pharmaceutical applications and professional system maintenance therefore ensure outstanding system quality and lasting value.
As the pioneer in the field of dry particle sizing technology, we have established ourselves on the international pharmaceutical market with numerous product-specific installations. World market leaders among the pharmaceutical research companies place their trust in our expertise, as do manufacturers of generic drugs and contract manufacturers as well as specialist companies with innovative niche products. Our quality management system has been repeatedly audited over the years and has been consistently tightened so that we do not just meet official directives and international standards but also demanding individual company standards.
Classic laser diffraction for particle size analysis in laboratory and process
The modular system concept of our proven HELOS laser diffraction sensors covers a very wide range of dry and wet applications through a number of dispersion and dosing options. Powders, granulates, solid and droplet aerosols as well as suspensions and emulsions are measured on a product-specific basis. For measurements in the submicron to millimetre range, our R series sets standards in terms of reproducibility, comparability and accuracy of particle size analysis – in combination with extremely short measuring times. The excellent reproducibility of dry dispersion with RODOS makes this system the first choice for all comminution stages and further dry process steps.
The laser diffraction systems of the MYTOS family provide in-process control, e.g. during continuous or batch-based micronisation of dry powders. In combination with innovative and representative samplers, we implement in-line and on-line systems which meet GMP requirements and can also be configured for use in explosion-hazard areas (ATEX). Use of the same technologies and identical components in both laboratory and manufacturing process areas guarantee maximum comparability of results when scaling up batch sizes.
Dynamic image analysis for characterisation of particle size and shape
Quality differences between batches with identical particle size distributions are frequently due to different particle shapes. Collective product properties such as flow behaviour, tabletting capability, adhesion or abrasion behaviour are directly related to the shape parameters of the individual particles. Dynamic image analysis with our modular QICPIC sensor combines characterisation of particle size and particle shape of powders, fibres, granulates or suspensions in the micron and millimetre range. A high-resolution, high-speed camera with up to 500 frames per second ensures to capture several million particles even in fast particle flows. Powerful algorithms supply meaningful, quantitative distributions of particle properties with high statistical confidence within a very short time. Illustrative, qualitative information on the individual particles is provided in the particle gallery.
The powerful components of the QICPIC system are also available for control and monitoring in the manufacturing process. The PICTOS family has been especially developed for dry and wet on-line applications and integrates dispersion and sensor technologies in a robust, GMP-compliant design.
Dynamic light scattering for analysis of concentrated nanodispersions
The application fields for particle size and stability analyses with dynamic light scattering (DLS) include suspensions and emulsions with nanoparticles. For example, ophthalmic agents or parenterals must meet specific requirements in relation to maximum particle size. Applications for nanomeasurement technology can also be found in active ingredient loading of innovative nano drug delivery systems. For the growing number of hardly soluble active ingredient candidates that are among the New Chemical Entities (NCE), nanosuspensions also offer good possibilities for development of formulations that improve bioavailability. Since the ambient parameters influence particle size growth and thus the stability of suspensions, undiluted samples should be used where possible in order to obtain meaningful results.
With dynamic light scattering, the scattered light intensities of thermally moved particles in suspension are evaluated to determine the particle size. In addition to conventional auto-correlation of the scattered light intensities (photon correlation spectroscopy (PCS)), our NANOPHOX DLS system also uses cross-correlation of two independent signals (photon cross-correlation spectroscopy (PCCS)). This allows suspensions with higher concentrations to be measured in their original condition without an elaborate dilution series.
Manufacture of pharmaceutical products is subject to strict national and international regulations. In order to obtain approval for a new drug, the manufacturer must first provide proof of efficacy, safety and quality to the responsible approval authorities. Correct and proper manufacture of approved pharmaceutical products is ensured by the Good Manufacturing Practice (GMP) guidelines. These require that pharmaceutical products and active ingredients (as well as cosmetics, food and animal feed) are produced under conditions that guarantee perfect product quality and also safety for patients and consumers. Quality must therefore be ensured by continuous monitoring during all production stages. While the starting materials are checked for perfect condition in the receiving inspection, batch-specific and non-batch-specific in-process control (IPC) measures are implemented during production.
These serve the purpose of monitoring and controlling the production process up to completion of the end product. The aim is to achieve fast, process-oriented monitoring and control so that problems can be detected early on and reject production avoided. Quality inspections are performed for each batch of the finished pharmaceutical product in the final inspection stage. In addition, complete documentation of all manufacturing steps and quality controls ensures subsequent traceability in the event of faults or complaints.
Our control and evaluation software supports implementation of the FDA (Food and Drug Administration) requirements stipulated in Part 11, Title 21 of the Code of Federal Regulations (21CFR11) on electronic records and signatures. The surface design of our instruments permits fast cleaning, prevents soiling by cross-contamination and therefore contributes to compliance with the GMP guidelines.
Industrially manufactured drugs are normally manufactured in batches. A batch is the production output of a quantity of a drug produced in a uniform manufacturing process. Comprehensive quality management with standard operating procedures (SOPs) is required in order to guarantee uniformity across batches – independently of the production location and operating personnel. Methods are standardised and validated in order to ensure that a method reliably produces the expected result within defined limits (specifications). Corresponding proof must be documented. Validation includes systematic checking of installations, equipment and processes to ensure that they are suitable for the intended purpose.
We provide a documented procedure in a validation binder for qualification of our measuring systems. This contains all the documentation for Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ). We also supply reference material for validation and re-certification of the overall system as part of PQ during ongoing system operation on the user's premises. This reference material is also used for quality assurance and certification during production of the measuring systems in our manufacture. Before a system is released with a certificate for customer operation, it is first subjected to comprehensive Production Operational Qualification (POQ) and Production Performance Qualification (PPQ). This is how we ensure to provide high-performing, precise and reliable systems with excellent repeatability and system-to-system comparability.